
[ad_1]
Connectomics, the formidable area of research that seeks to map the intricate community of animal brains, is present process a development spurt. Throughout the span of a decade, it has journeyed from its nascent phases to a self-discipline that’s poised to (hopefully) unlock the enigmas of cognition and the bodily underpinning of neuropathologies akin to in Alzheimer’s illness.
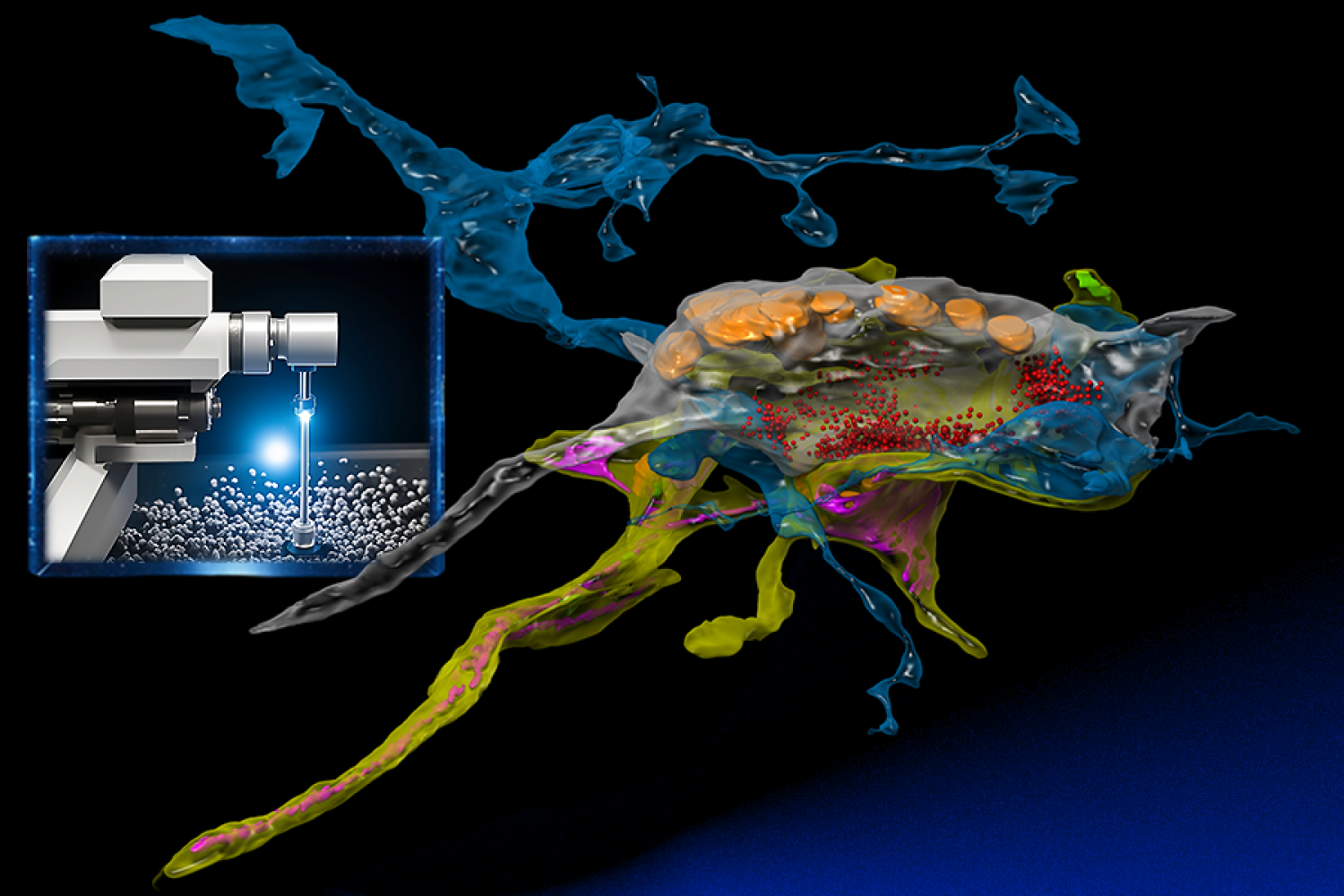
At its forefront is the usage of highly effective electron microscopes, which researchers from the MIT Pc Science and Synthetic Intelligence Laboratory (CSAIL) and the Samuel and Lichtman Labs of Harvard College bestowed with the analytical prowess of machine studying. In contrast to conventional electron microscopy, the built-in AI serves as a “mind” that learns a specimen whereas buying the pictures, and intelligently focuses on the related pixels at nanoscale decision just like how animals examine their worlds.
“SmartEM” assists connectomics in shortly analyzing and reconstructing the mind’s complicated community of synapses and neurons with nanometer precision. In contrast to conventional electron microscopy, its built-in AI opens new doorways to know the mind’s intricate structure.
The combination of {hardware} and software program within the course of is essential. The group embedded a GPU into the help laptop linked to their microscope. This enabled operating machine-learning fashions on the pictures, serving to the microscope beam be directed to areas deemed attention-grabbing by the AI. “This lets the microscope dwell longer in areas which are more durable to know till it captures what it wants,” says MIT professor and CSAIL principal investigator Nir Shavit. “This step helps in mirroring human eye management, enabling fast understanding of the pictures.”
“Once we have a look at a human face, our eyes swiftly navigate to the focal factors that ship important cues for efficient communication and comprehension,” says the lead architect of SmartEM, Yaron Meirovitch, a visiting scientist at MIT CSAIL who can also be a former postdoc and present analysis affiliate neuroscientist at Harvard. “Once we immerse ourselves in a guide, we do not scan the entire empty house; quite, we direct our gaze in direction of the phrases and characters with ambiguity relative to our sentence expectations. This phenomenon inside the human visible system has paved the best way for the start of the novel microscope idea.”
For the duty of reconstructing a human mind phase of about 100,000 neurons, attaining this with a traditional microscope would necessitate a decade of steady imaging and a prohibitive finances. Nevertheless, with SmartEM, by investing in 4 of those revolutionary microscopes at lower than $1 million every, the duty may very well be accomplished in a mere three months.
Nobel Prizes and little worms
Over a century in the past, Spanish neuroscientist Santiago Ramón y Cajal was heralded as being the primary to characterize the construction of the nervous system. Using the rudimentary mild microscopes of his time, he launched into main explorations into neuroscience, laying the foundational understanding of neurons and sketching the preliminary outlines of this expansive and uncharted realm — a feat that earned him a Nobel Prize. He famous, on the subjects of inspiration and discovery, that “So long as our mind is a thriller, the universe, the reflection of the construction of the mind can even be a thriller.”
Progressing from these early phases, the sphere has superior dramatically, evidenced by efforts within the Nineteen Eighties, mapping the comparatively easier connectome of C. elegans, small worms, to at present’s endeavors probing into extra intricate brains of organisms like zebrafish and mice. This evolution displays not solely huge strides, but in addition escalating complexities and calls for: mapping the mouse mind alone means managing a staggering thousand petabytes of information, a process that vastly eclipses the storage capabilities of any college, the group says.
Testing the waters
For their very own work, Meirovitch and others from the analysis group studied 30-nanometer thick slices of octopus tissue that had been mounted on tapes, placed on wafers, and eventually inserted into the electron microscopes. Every part of an octopus mind, comprising billions of pixels, was imaged, letting the scientists reconstruct the slices right into a three-dimensional dice at nanometer decision. This supplied an ultra-detailed view of synapses. The chief intention? To colorize these photos, determine every neuron, and perceive their interrelationships, thereby creating an in depth map or “connectome” of the mind’s circuitry.
“SmartEM will minimize the imaging time of such initiatives from two weeks to 1.5 days,” says Meirovitch. “Neuroscience labs that at present cannot be engaged with costly and lengthy EM imaging will have the ability to do it now,” The tactic must also enable synapse-level circuit evaluation in samples from sufferers with psychiatric and neurologic issues.
Down the road, the group envisions a future the place connectomics is each reasonably priced and accessible. They hope that with instruments like SmartEM, a wider spectrum of analysis establishments might contribute to neuroscience with out counting on massive partnerships, and that the tactic will quickly be a regular pipeline in instances the place biopsies from residing sufferers can be found. Moreover, they’re keen to use the tech to know pathologies, extending utility past simply connectomics. “We at the moment are endeavoring to introduce this to hospitals for giant biopsies, using electron microscopes, aiming to make pathology research extra environment friendly,” says Shavit.
Two different authors on the paper have MIT CSAIL ties: lead writer Lu Mi MCS ’19, PhD ’22, who’s now a postdoc on the Allen Institute for Mind Science, and Shashata Sawmya, an MIT graduate pupil within the lab. The opposite lead authors are Core Francisco Park and Pavel Potocek, whereas Harvard professors Jeff Lichtman and Aravi Samuel are extra senior authors. Their analysis was supported by the NIH BRAIN Initiative and was offered on the 2023 Worldwide Convention on Machine Studying (ICML) Workshop on Computational Biology. The work was carried out in collaboration with scientists from Thermo Fisher Scientific.
[ad_2]