
[ad_1]
Abstract: A brand new examine reveals the important perform of the protein Kdm1a in sustaining neuronal id and stopping the untimely ageing of neurons. Via experiments in mice and corroborated by human knowledge, researchers discovered that Kdm1a suppresses inappropriate gene expression, preserving the epigenetic boundaries that distinguish energetic and inactive chromatin areas.
The lack of Kdm1a leads to the disruption of those boundaries and the activation of genes that ought to stay silent, mirroring the consequences of pure ageing and contributing to neurological issues. This analysis underscores the significance of epigenetic regulation in neuronal well being and gives insights into the mechanisms of ageing and illness.
Key Details:
- The protein Kdm1a is important for preserving the id of neurons by repressing genes that shouldn’t be expressed, highlighting its position in epigenetic regulation.
- Lack of Kdm1a mirrors ageing processes by activating inappropriate gene expression, suggesting its involvement in neuronal ageing and potential mental disabilities.
- The examine, supported by vital funding sources, combines mouse mannequin analysis with human knowledge evaluation, demonstrating the common relevance of Kdm1a in sustaining neuronal integrity throughout species.
Supply: UMH
Epigenetic processes permit completely different cell varieties to emerge from a single genome. All through improvement, cells differentiate and purchase distinct traits by expressing the identical genome in several methods. Nevertheless, a less-known facet of this course of is how cells preserve their distinctive identities over time.
A examine led by the Transcriptional and Epigenetic Mechanisms of Neuronal Plasticity laboratory, headed by Angel Barco on the Institute for Neurosciences, a joint middle of the Spanish Nationwide Analysis Council (CSIC) and the Miguel Hernández College (UMH) of Elche, has decided that the protein Kdm1a performs a vital position in preserving the id of neurons.
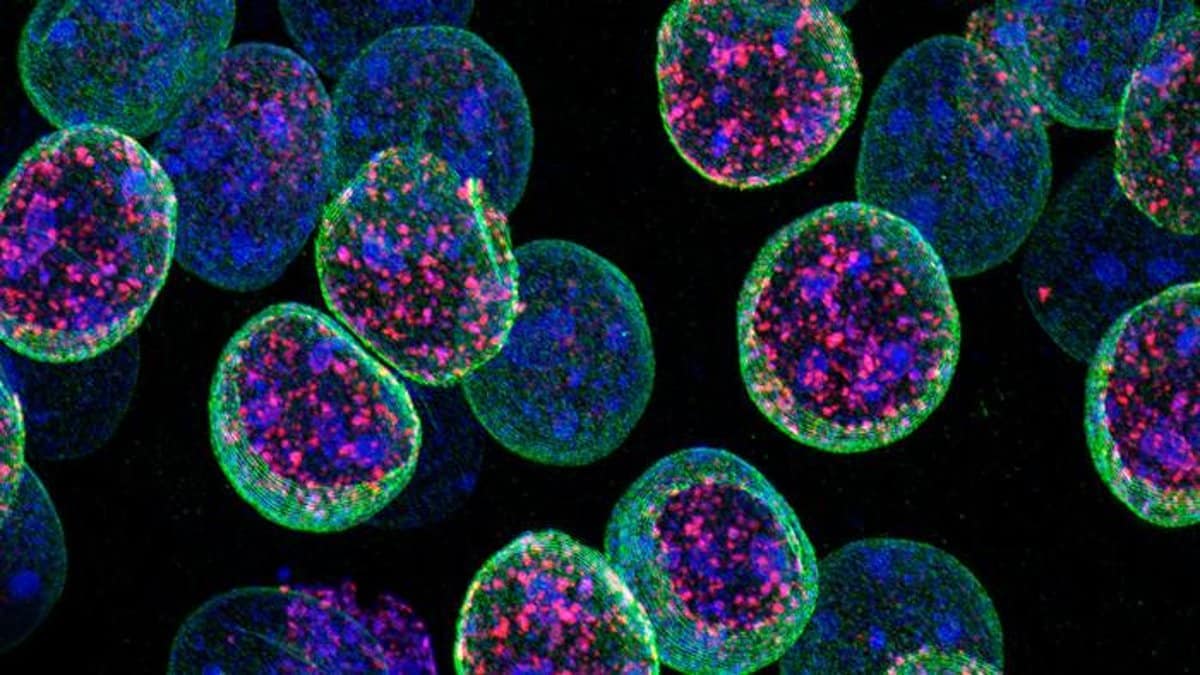
Neurons are cells which have a really explicit nuclear construction as a result of as soon as they’re shaped throughout improvement they by no means divide once more, which is why neurons want to exactly preserve their id all through their lifespan.
“Id is given by what is finished and what’s not completed; at an epigenetic degree this interprets into the genes which are expressed, but additionally in these that aren’t expressed” explains Angel Barco.
The outcomes of this examine, revealed within the journal Nature Communications, exhibit that deleting Kdm1a in forebrain neurons in grownup mice triggers the expression of genes that usually shouldn’t be expressed in neurons, which compromises neuronal id.
The researchers verified that in regular aged mice, there may be an incipient activation of the identical genes derepressed in mice which have misplaced Kdm1a, indicating that pure ageing reproduces the identical defects as the shortage of Kdm1a, though on a smaller scale.
They discovered that eliminating this protein accelerates neuronal ageing at an epigenetic degree and alters gene transcription.
The consultants correlated these findings with human knowledge by collaborating with researcher José Vicente Sánchez Mut, who leads the Useful Epi-Genomics of Getting older and Alzheimer’s Illness laboratory on the IN. They used a database of individuals between 50 and 80 years outdated and located that there’s additionally a major enhance within the expression of a few of these sometimes silent genes as folks age.
Moreover, this work exhibits that the repressive perform of Kdm1a maintains the separation between genes that must be expressed and people who must be silent by the modulation of chromatin construction.
The separation into “compartments” permits the upkeep of order all through the lifespan of the neuron, which is important to protect its id: “We all know that dysfunction can have detrimental results, each in ageing and in mental incapacity as a result of it blurs the barrier between what must be expressed and what mustn’t,” says Beatriz del Blanco, first creator of the article.
To know the position of Kmd1a in compartmentalization, the researchers carried out an experiment, in collaboration with Yijun Ruan, former director of the Jackson Laboratory for Genomic Drugs, wherein they used a method that exhibits how DNA folds contained in the cell nucleus, and the way it’s organized in three dimensions.
These knowledge have been contrasted with photos obtained utilizing super-resolution microscopy. Each approaches indicated that some repressed genes started to be expressed as a result of the boundaries that delimit energetic and inactive areas of the chromatin are weakened after Kdm1a loss.
The laboratory directed by Barco on the IN investigates uncommon neurological issues linked to mutations in genes encoding epigenetic regulators. Mutations within the Kmd1a protein throughout improvement have been related to an especially uncommon mental incapacity dysfunction generally known as Cleft Palate, Psychomotor Delay, Distinctive Facial Options, and Mental Incapacity (CPRF Syndrome). Angel Barco emphasizes the significance of learning uncommon ailments to develop the bounds of data on this area.
Funding: This work has been potential because of funding from the Spanish Analysis Company-Ministry of Science, Innovation, and Universities and the Fundació la Marató de TV3, amongst different sources.
About this neuroscience analysis information
Writer: Angeles Gallar
Supply: UMH
Contact: Angeles Gallar – UMH
Picture: The picture is credited to Barco, A. Instituto de Neurociencias UMH-CSIC
Authentic Analysis: Open entry.
“Kdm1a safeguards the topological boundaries of PRC2-repressed genes and prevents aging-related euchromatinization in neurons” by Barco, A et al. Nature Communications
Summary
Kdm1a safeguards the topological boundaries of PRC2-repressed genes and prevents aging-related euchromatinization in neurons
Kdm1a is a histone demethylase linked to mental incapacity with important roles throughout gastrulation and the terminal differentiation of specialised cell varieties, together with neurons, that is still extremely expressed within the grownup mind.
To discover Kdm1a’s perform in grownup neurons, we develop inducible and forebrain-restricted Kdm1a knockouts.
By making use of multi-omic transcriptome, epigenome and chromatin conformation knowledge, mixed with super-resolution microscopy, we discover that Kdm1a elimination causes the neuronal activation of nonneuronal genes which are silenced by the polycomb repressor complicated and interspersed with energetic genes.
Useful assays exhibit that the N-terminus of Kdm1a incorporates an intrinsically disordered area that’s important to segregate Kdm1a-repressed genes from the neighboring energetic chromatin atmosphere.
Lastly, we present that the segregation of Kdm1a-target genes is weakened in neurons throughout pure ageing, underscoring the position of Kdm1a safeguarding neuronal genome group and gene silencing all through life.
[ad_2]